Temperature and heat are two distinct physical properties. Heat is a substance’s total amount of energy, whereas temperature is the average kinetic energy of its particles. This article will go over the differences between heat and temperature in great detail.
What is Heat?
Heat is a type of energy transfer that occurs between two objects with different temperatures. It is a flow of energy from a higher temperature object to a lower temperature object. Heat is a type of kinetic energy, or motion energy. It is a type of energy that is transferred from one body to another as a result of a temperature difference. Heat is typically transmitted via conduction, convection, or radiation.
Heat is transferred through direct contact between molecules in conduction. When two objects come into contact with each other, heat energy is transferred between them. The heat from the hotter object is absorbed by the colder object. It is transferred by convection when a fluid, such as air or water, moves. The heat is carried by the fluid as it moves. This is the primary mode of heat transfer in the atmosphere.
Also Read > Difference Between Mass and Weight
The transfer of heat energy through space is known as radiation. Electromagnetic waves are used to transfer heat. In a vacuum, the only way to transfer heat is through radiation. It is an essential part of our daily lives. It is used to heat structures and cook food. It’s also used to power engines and generate electricity. In industrial processes, heat is also used to change the physical state of materials.
What is Temperature?
Temperature is a measure of a particle system’s average kinetic energy. The concept of temperature is closely related to the concept of heat, and it is used to quantify a system’s thermal energy. Temperature is usually measured on a temperature scale in degrees Celsius, Fahrenheit, or Kelvin. Temperature is an important physical quantity because it represents a system’s average kinetic energy. This is important for many physical processes, including gas behaviour, chemical reaction rates, and heat flow.
It also has an impact on the properties of substances, such as their boiling and melting points. Heat is defined as the energy transferred between two objects as a result of a temperature difference. If one object has a higher temperature than the other, heat will flow from the higher temperature object to the lower temperature object.
Thermal conduction is the name given to this process. Radiation, which is the emission of electromagnetic energy, can also be used to transfer heat. The presence of other objects, such as the Sun or other sources of energy, influences temperature. The amount of material in a system, as well as its chemical composition, influence temperature. The pressure and volume of the system also have an effect on the temperature.
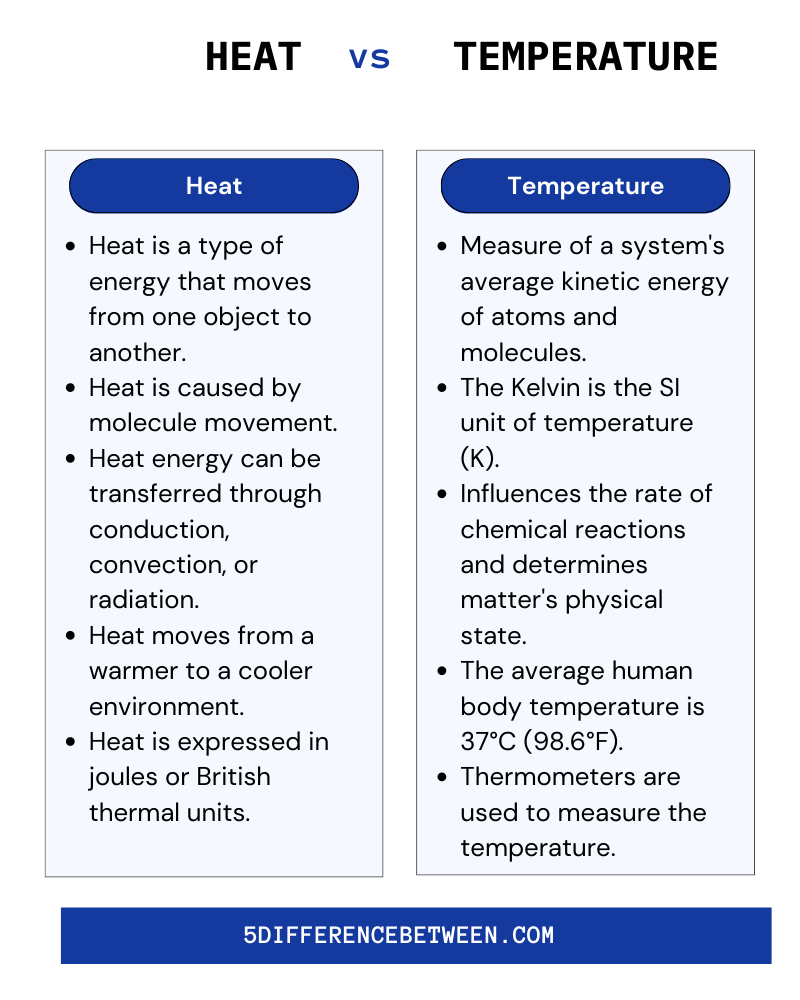
Heat Vs Temperature
Effects of Heat and Temperature on Human Beings
- The total amount of energy present is referred to as heat, whereas temperature is the average kinetic energy of the particles in a system. Prolonged exposure to high temperatures can cause heat exhaustion, heat stroke, and dehydration. Heat exhaustion is caused by electrolyte and fluid loss, and it can result in dizziness, weakness, and even fainting. Heat stroke is a more serious condition that occurs when the body temperature rises above 104°F.
- It has the potential to cause dizziness, seizures, and even death. Dehydration occurs when the body does not have enough water or electrolytes and can result in headaches, fatigue, and confusion. Heat causes physical discomforts such as muscle cramps, dizziness, and headaches. Heat can irritate the skin and eyes, as well as cause sunburn. Extreme cold temperatures, on the other hand, can be hazardous to one’s health.
- Hypothermia occurs when the body temperature falls below 95 degrees Fahrenheit. Among the symptoms are confusion, shivering, and exhaustion. Frostbite is another condition that can occur when exposed to cold temperatures. Numbness, skin reddening, and tissue damage are all symptoms. Drinking plenty of fluids, wearing appropriate clothing, and limiting time spent in extreme temperatures are all important precautions.
The average kinetic energy of the molecules is measured by temperature, whereas heat is the total energy of all the molecules in a system. Heat is measured in Joules, whereas temperature is measured in degrees Celsius. Heat is a type of energy, whereas temperature is a measurement of the average kinetic energy of molecules. Heat can change the temperature, but heat cannot change the temperature. Finally, while both are related concepts, they have distinct properties and measurements.






One Comment to “5 Difference Between Heat and Temperature”