Compounds and mixtures are two frequently used chemistry terms. They are both made up of two or more substances, but the way the substances are combined and interact with one another differentiates them. Compounds are molecules that are chemically bonded together, whereas mixtures are molecules that are physically combined but are not chemically bonded. This article will go into greater detail about the difference between compound and mixture.
The Essential Difference Between Compound and Mixture
To understand in detail the difference between compound and mixture it is truly essential to understand what they are.
What is a Compound?
A compound is a substance that results from the chemical combination of two or more elements in a specific ratio. Organic and inorganic compounds can coexist. Water (H2O), table salt (NaCl), carbon dioxide (CO2), and methane are examples of compounds (CH4). Organic compounds are primarily composed of carbon and hydrogen, but they may also contain oxygen, nitrogen, sulfur, phosphorus, and halogens. Organic compounds can be both natural and man-made. Proteins, carbohydrates, lipids, and nucleic acids are some of the most common organic compounds found in nature.
Many synthetic organic compounds are also found in commonplace items like plastics and pharmaceuticals. Inorganic compounds, on the other hand, do not contain carbon. They are composed of elements like sulfur, nitrogen, oxygen, and metals. Common inorganic compounds include sulfuric acid (H2SO4), table salt (NaCl), ammonia (NH3), and carbon dioxide (CO2). Cleaning acids and bases, as well as agricultural fertilizers, are examples of inorganic compounds that are commonly used in industry. Compounds are typically made up of atoms that are held together by chemical bonds.
Also Read > Difference Between Concave and Convex Mirror
These bonds can be covalent (two atoms sharing electrons) or ionic (two atoms sharing protons) (one atom giving up electrons to another atom). Depending on the type of bond, compounds can be soluble or insoluble in water. Compounds are necessary for a variety of functions in our daily lives. They are used as building blocks in construction, as medicine in pharmaceuticals, and as fuel in energy sources. Compounds are essential to life as we know it.
What is a Mixture?
A mixture is a physical combination of two or more non-chemically combined substances. There can be homogeneous or heterogeneous mixtures. The components of a homogeneous mixture are evenly distributed and have the same properties throughout. A solution is an example of a homogeneous mixture because it is made up of a solute dissolved in a solvent. A heterogeneous mixture is made up of components that are unevenly distributed and have varying properties throughout.
A salad is an example of a heterogeneous mixture because it contains discernible lettuce, tomatoes, onions, and carrots. There are numerous techniques for breaking down mixtures into their constituents. Filtration can be used to separate a solid and a liquid mixture, such as coffee grounds and water. As the liquid passes through the filter, the solid particles become trapped. For example, the distillation process is used to separate two or more liquids with different boiling points. The liquid with a lower boiling point evaporates first, then condenses back into a liquid. Physical properties can also be used to classify mixtures. They could be gaseous, liquid, or solid, for example.
Gaseous mixtures are made up of two or more gasses, for example, oxygen and nitrogen. Liquid mixtures are composed of two or more liquids, such as water and oil. Solid mixtures are composed of two or more solids, such as sugar and sand. Nature and everyday life are full of mixtures. Soil is a mineral, organic matter, water, and air mixture, whereas air is a gas mixture. Many commonplace items, such as paint, shampoo, and perfume, contain a variety of ingredients. Many industrial processes, such as the production of chemicals, pharmaceuticals, and food products, rely on mixtures.
Compound Vs Mixture
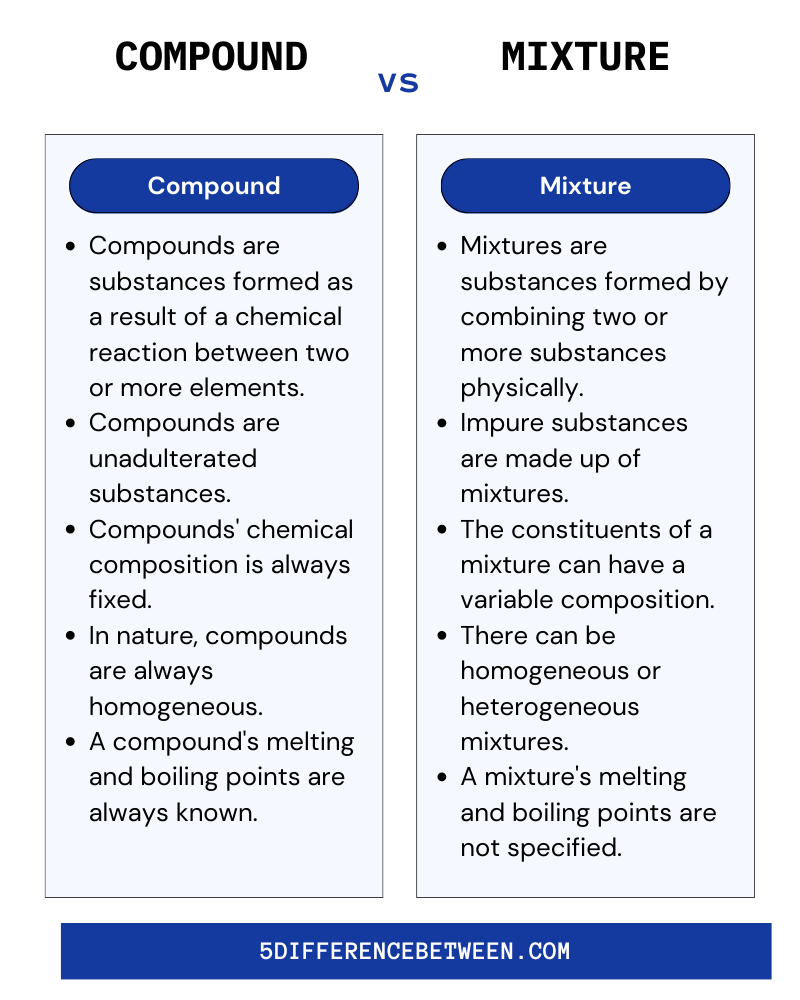
These are the primary distinctions between mixtures and compounds that must be understood for these two.






One Comment to “5 Difference Between Compound and Mixture”