You’ve presumably run over acetic acid or acetic anhydride eventually, whether in a secondary school chemistry class or a Do-It-Yourself science experiment. While their names might sound comparable, these two chemicals have a few key contrasts you ought to be aware of. Acetic acid is a dreary natural compound that gives vinegar its harsh taste and impactful smell. Acetic anhydride is an acid anhydride that is used to convey cellulose acetic acid deduction, which is found in items like visual film and materials.
Anyway, both derived from the acetic acid molecule, and their chemical structures and properties are exceptionally novel.
Chemical Structure
Acetic acid is a characteristic acid made of carbon, hydrogen and oxygen atoms. It contains a carboxyl group which gives it acidic properties. It is composed of two acetic acid derivation groups combined, with a deficiency of water. So you could say acetic anhydride is a dried-out type of acetic acid.
As a result of their various structures, both have unmistakable properties. Acetic acid is a destructive fluid that produces hydrogen particles in water, making it acidic. Acetic anhydride is a drab fluid with an impactful smell. It is profoundly responsive and utilized as an acetylating specialist to create esters and amides for different natural combinations.
Though they are connected mixtures, they have huge contrasts in their chemical cosmetics and properties. Acetic acid is a natural acid, acetic anhydride is an acid anhydride. Acetic acid is acidic, acetic anhydride is exceptionally receptive. Knowing these distinctions is key to understanding how these useful chemicals are utilized in various industrial applications.
Also Read > Difference Between Love and Admiration
Reactivity and Uses
Both useful chemicals, but their reactivity and applications differ quite a bit. Acetic acid, also known as vinegar, is great for pickling and preserving foods. It’s edible and safe in small doses, with a distinctive sour taste. Acetic anhydride, on the other hand, is highly reactive and toxic, used mainly for industrial purposes.
- Uses
Acetic acid is widely used as a food additive, natural preservative, and cleaning agent. In the lab, it’s useful for adjusting pH and as a solvent. Acetic anhydride is employed to produce cellulose acetate for photographic films, fibers, and plastics. It also finds use in the synthesis of aspirin and other pharmaceuticals.
- Reactivity
Acetic acid is a weak acid that to some degree ionizes in water. It’s reactive but less so than acetic anhydride, which is highly corrosive. Acetic anhydride responds energetically to water and alcohol, so should be handled cautiously.
The two compounds are natural particles containing carbon, hydrogen, and oxygen atoms. Notwithstanding, acetic anhydride has an additional acetic derivation group that makes it more electrophilic and inclined to respond with nucleophiles.
In synopsis, while both share some properties, their reactivity, toxicity, and applications can contrast significantly. Knowing the vital contrasts between these two chemicals is significant for utilizing them appropriately and securely.
Acetic Acid vs Acetic Anhydride
Acetic acid and acetic anhydride are two combinations derived from acetic acid but have some vital differences in their chemical structure and properties.
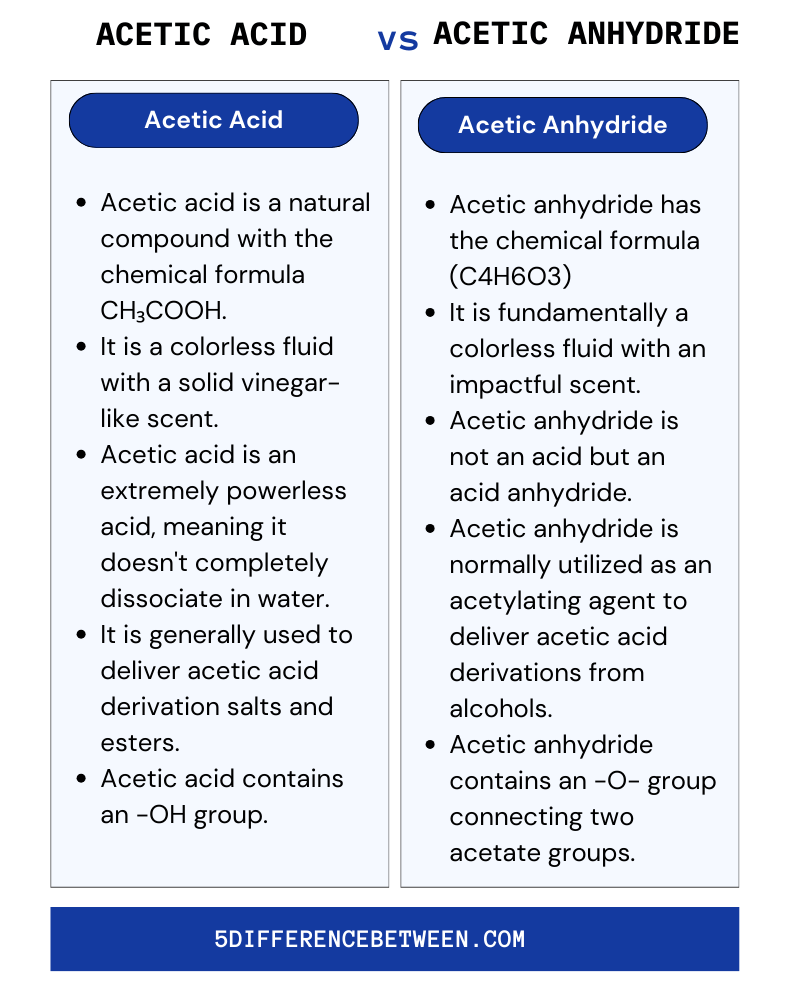
Acetic Acid
- Acetic acid is a natural compound with the chemical formula CH₃COOH.
- It is a colorless fluid with a solid vinegar-like scent.
- Acetic acid is an extremely powerless acid, meaning it doesn’t completely dissociate in water.
- It is generally used to deliver acetic acid derivation salts and esters.
- Acetic acid contains an -OH group.
Acetic Anhydride
- Acetic anhydride has the chemical formula (C4H6O3)
- It is fundamentally a colorless fluid with an impactful scent.
- It can respond with water to create acetic acid.
- Normally utilized as an acetylating agent to deliver acetic acid derivations from alcohols.
- Acetic anhydride contains an -O- group connecting two acetate groups.
While both are firmly related, understanding the distinctions in their chemical properties and reactivity is vital to utilizing them appropriately for different applications. With this information, you’ll be well-headed to turning into a vinegar or acetic acid derivation master!






