Have you at any point considered the things that differ between graphite and lead? You probably use pencils regularly and have heard the terms “graphite pencil” and “lead pencil” used correspondingly. Regardless, is there actually a differentiation, or are those terms referring to the very same thing? There’s something else to the pencil besides what might be expected. While graphite and lead pencils might seem comparative, they contain various materials at their center literally. Read on to finally understand the difference once and for all.
What Is Graphite
Graphite is a normally occurring type of carbon. It consists of carbon molecules organized in sheets of hexagonal lattices stacked on top of one another. Between these sheets are weak bonds, permitting the sheets to slide past one another. This is what gives graphite its soft, slippery feel.
Also Read > Difference Between Tortilla and Chapati
Graphite is made of carbon, it has a lesser thickness and is poisonous to humans. Graphite is non-harmful and chemically inert. It will not respond to or dissolve in water and most chemicals. The softness and slipperiness of graphite is what makes it useful for applications like:
- Pencils. Graphite’s sheets slide past each other, depositing thin layers onto paper. By blending graphite in with clay, pencil lead of various hardnesses can be delivered.
- Lubricants. Graphite’s elusive quality is valuable as a dry grease for locks, hinges, and other sliding surfaces.
- Steel production. Graphite is utilized in steelmaking to reduce impurities and as a grease.
- Batteries. Graphite’s capacity to lead electricity and endure high intensity makes it helpful as an anode material in lithium-ion batteries.
- Nuclear reactors. Graphite is utilized as a mediator to slow down neutrons in some atomic reactor plans.
What Is Lead
Lead is an ordinarily occurring weighty metallic that has been used by individuals for pretty a long time. Its substance symbol is Pb, which comes from plumbum, the Latin word for lead. You’re logically familiar with lead as a fixing in pencils, batteries, and pipes, yet it has various purposes as well.
Lead is highly harmful to people, particularly to kids, so many of its purposes have been banned or limited. However, its thickness, low liquefying point, and resistance to corrosion made it valuable for applications like pipes, pencils, and batteries. Lead pencils contain graphite, not natural lead, but their name comes from the lead packaging that was originally utilized. Lead-acid batteries, similar to those utilized in vehicles, contain lead plates and lead dioxide, which respond to produce power.
Application
However lead was once generally used in paint, fuel, and plumbing, we currently know that any exposure to lead can be dangerous. Ingesting or breathing in lead particles, particularly at a young age, can cause long-lasting harm to the brain and nervous system. Therefore, lead paint and leaded fuel were banned, and lead lines and solder were ended.
Any metal with similar chemical properties could potentially replace lead in most applications. Dense metals like bismuth or tin can substitute for lead in weights and radiation shielding. Copper or plastic pipes are more secure choices for plumbing. Lithium-ion and nickel-metal hydride batteries can supplant lead-acid batteries. Graphite and polymers are currently utilized in many pencils.
However lead will probably continue to be utilized in a few industrial applications, decreasing or eliminating its utilization in consumer items guarantees that individuals are not exposed to its harmful impacts, particularly weak groups like kids. More secure, lead-free options ought to be utilized whenever the situation allows.
In short, graphite and lead might sound comparable, however, they have very different properties and utilization. Graphite is a flexible and valuable type of carbon, while lead is a thick, poisonous metal. Ideally, this clears up any disarray between these two materials!
Graphite vs Lead
Graphite and lead are frequently mistaken for each other, yet they really have a few major differences. Here are five key ways graphite and lead differ:
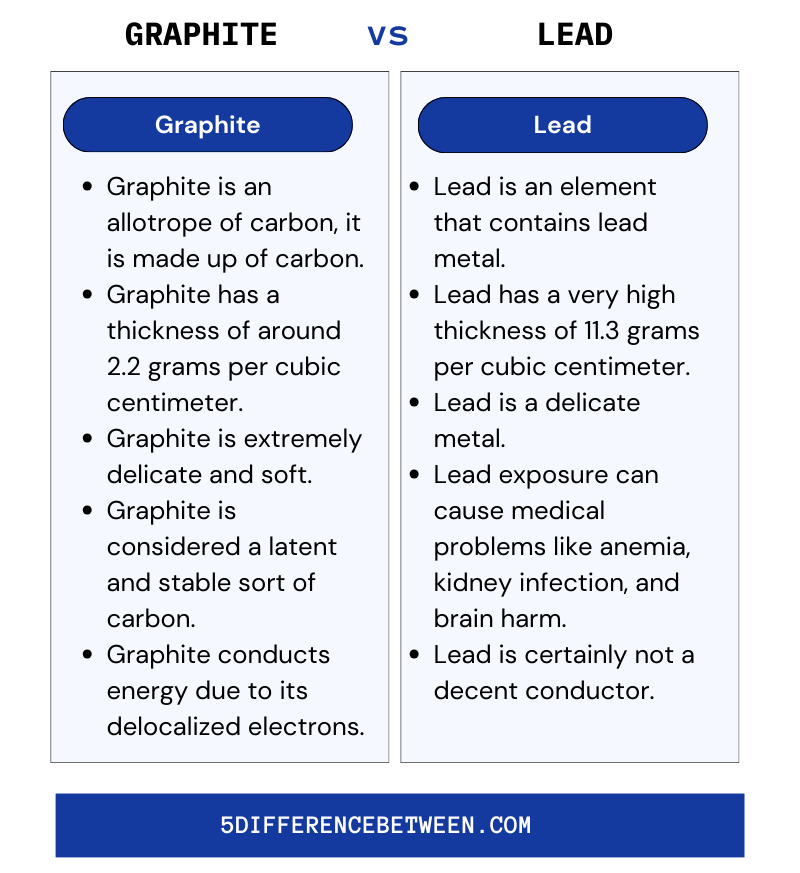
Graphite
- Graphite is an allotrope of carbon, it is made up of carbon.
- Graphite has a thickness of around 2.2 grams per cubic centimeter.
- Graphite is extremely delicate and soft.
- Graphite is considered a latent and stable sort of carbon.
- Graphite conducts energy due to its delocalized electrons.
Lead
- Lead is an element that contains lead metal.
- Lead has a very high thickness of 11.3 grams per cubic centimeter.
- Lead is a delicate metal.
- Lead exposure can cause medical problems like anemia, kidney infection, and brain harm.
- Lead is certainly not a decent conductor.
In summary, while graphite and lead are frequently confused with each other because of their delicate, gray nature and use in pencils, they have a few huge contrasts in their composition, density, softness, harmfulness, and electrical conductivity.