You have surely heard about varieties of solvents in your science classes, yet do you have any idea what precisely sets protic and aprotic solvents apart? While they both dissolve things, the important differences between these two solvent groups give you a deeper grasp of why chemists select one over the other for positive reactions and solutions.
Defining Protic and Aprotic Solvents
Protic solvents include hydrogens that can shape hydrogen bonds with solutes. Some common examples of protic solvents include water, methanol, and also ethanol. These mentioned solvents are polar and capable of dissolving other polar molecules. Their hydrogen bonding means they regularly stabilize ions or maintain ionic compounds in solution.
Aprotic solvents need hydrogens which could take part in hydrogen holding. Solvents like acetone, dimethylformamide (DMF), and dimethyl sulfoxide (DMSO) are regular aprotic solvents. They are not able to structure hydrogen bonds but are still polar enough to dissolve polar and ionic compounds.
The properties of protic vs. aprotic solvents lead to some key differences in their conduct:
- Protic solvents are higher at solvating cations owing to the fact they can surround the positive charge with their hydrogen bonds. Aprotic solvents have a harder time solvating cations because of the absence of hydrogen holding.
- Reactions in protic solvents commonly maintain at a slower rate than in aprotic solvents. The hydrogen bonds can stabilize transition states and reactants, raising the activation energy required for a response.
- Protic solvents are extra acidic than aprotic solvents and can take part as a proton donor in acid-base reactions. Aprotic solvents are very inert.
In the end, the selection of a protic or aprotic solvent comes down to the particular needs of your reaction or test. Both have useful properties, so figuring out which hydrogen bonding capability is more important can guide you to the proper solvent system.
Also Read > Difference Between Final and Finale
Examples of Common Protic and Aprotic Solvents
Let’s start with some common protic solvents:
- Water (H2O)
The most familiar protic solvent is water. It’s polar, meaning it has separated positive and negative ends, and forms hydrogen bonds. This allows it to dissolve different polar and ionic compounds.
- Ammonia (NH3)
Ammonia is another regular protic solvent utilized in different applications like the assembling of manures and explosives. It has one nitrogen particle and three hydrogen molecules, giving it the system NH3.
- Methanol (CH3OH)
Methanol, also called methyl liquor, is the most basic alcohol and a comprehensively utilized protic solvent. It has a hydroxyl group (OH) which could give protons, and is as often as possible utilized as a solvent for organic responses and to make biodiesel fuel.
Some well-known aprotic solvents include:
- Acetone ((CH3)2CO)
Acetone is a common aprotic solvent utilized in nail paint remover and cleaning things. it’s a 3-carbon molecule with two methyl groups and one carbonyl group. As it lacks protons to give, acetone can’t shape hydrogen bonds.
- Dimethylformamide (DMF) ((CH3)2NC(O)H)
DMF is a polar aprotic solvent often used in polymer creation and pharma production. It has extreme solvency power, dissolving both polar and nonpolar blends.
- Hexane (C6H14)
Hexane is a non-regular aprotic solvent obtained from unrefined petroleum. It’s rarely utilized in fluid and chromatography. Hexane lacks polarity, so it won’t dissolve ionic or polar substances. But it works great for dissolving nonpolar compounds.
- Chloroform (CHCl3)
Chloroform was once utilized as a sedative, however today it serves principally as an aprotic solvent. It’s denser than water and breaks down fats, waxes, and alkaloids. Chloroform lacks major hydrogen particles, so it can’t give or acknowledge protons.
Protic vs Aprotic Solvents
Protic and aprotic solvents are two broad classes of solvents that contrast in a few significant ways. The following are five of the major differences to comprehend.
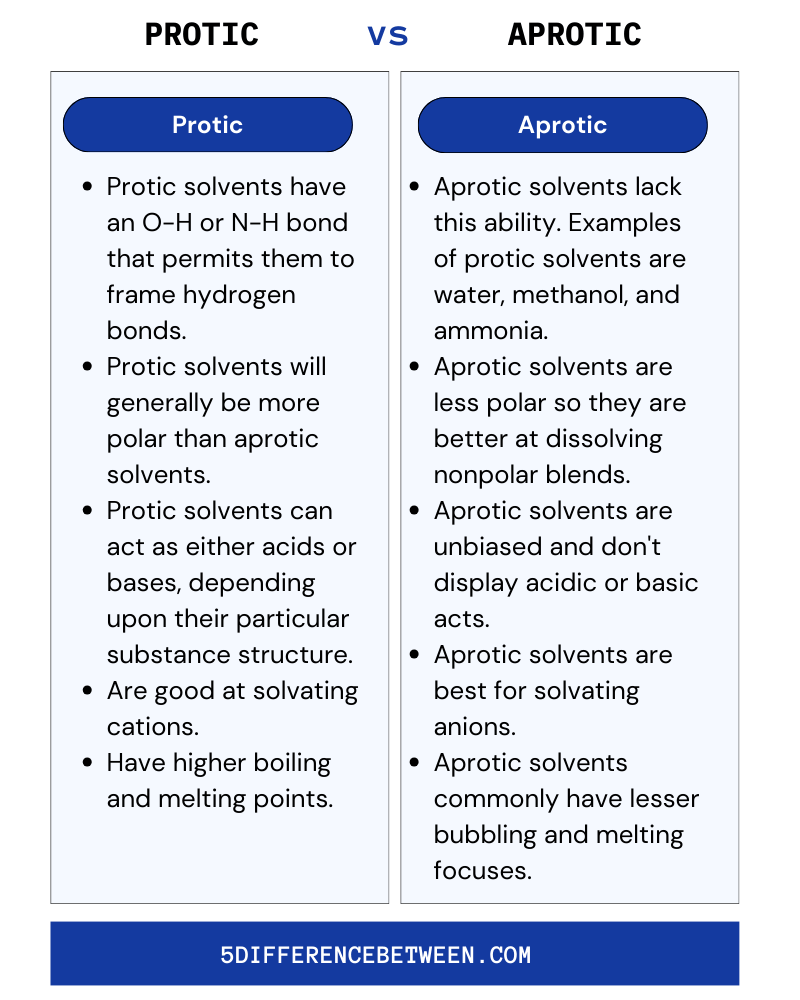
Protic
- Protic solvents have an O-H or N-H bond that permits them to frame hydrogen bonds.
- Protic solvents will generally be more polar than aprotic solvents which makes them great at dissolving other polar and ionic mixtures.
- Protic solvents can act as either acids or bases, depending upon their particular substance structure.
- Protic solvents are good at solvating cations.
- Protic solvents typically have higher boiling and melting points.
Aprotic
- Aprotic solvents lack this ability. Examples of protic solvents are water, methanol, and ammonia.
- Aprotic solvents are less polar so they are better at dissolving nonpolar blends.
- Aprotic solvents are unbiased and don’t display acidic or basic acts.
- Aprotic solvents are best for solvating anions.
- Aprotic solvents commonly have lesser bubbling and melting focuses.
In summary, the capacity to form hydrogen bonds, polarity, acidic/basic nature, solvation capacity, and physical properties are the absolute best ways protic and aprotic solvents vary from one another. Understanding these distinctions can help in choosing the proper solvent for your application.