Osmosis and diffusion are two processes that organisms require to function properly. Both processes involve molecules moving from high to low concentration zones. Despite their similarities, the two processes are vastly different. It is essential to understand the difference between osmosis and diffusion for a better iq.
What is Osmosis and How Does It Work?
Osmosis is the process by which molecules of a solvent, such as water, move through a semipermeable membrane from a region of higher concentration to a region of lower concentration. It is crucial in the regulation of water balance in living cells. Osmosis is required for the transport of nutrients, waste, and other substances into and out of cells. It is also required for water absorption from the digestive tract and proper kidney function.
Also Watch > Difference Between Mitosis and Meiosis
Osmosis is required for cell homeostasis to be maintained. It is in charge of transporting water and other solutes across cell membranes, which allows cells to maintain internal balance. Moving water from a higher to a lower concentration can help to equalize solute concentrations in cells, resulting in a more stable environment. Osmosis also aids in the regulation of the body’s water balance by allowing water to move as needed between tissues and organs.
What is Diffusion and How Does It Work?
The movement of molecules from a high concentration area to a low concentration area is referred to as diffusion. This process, which is important in many biological processes, is driven by the kinetic energy of the molecules. Diffusion is the primary mechanism for transporting oxygen and other molecules throughout the body and is required for substance transport across cell membranes. Diffusion is also important in cell metabolism because molecules move from one cellular compartment to another in order to produce energy.
Diffusion also affects the rate of chemical reactions within the body. As molecules move from a high concentration area to a low concentration area, the reaction rate increases. This is due to the fact that the molecules needed for the reaction are more likely to be present at the same time. As a result, diffusion is crucial in the regulation of physiological processes all over the body.
Osmosis Vs Diffusion
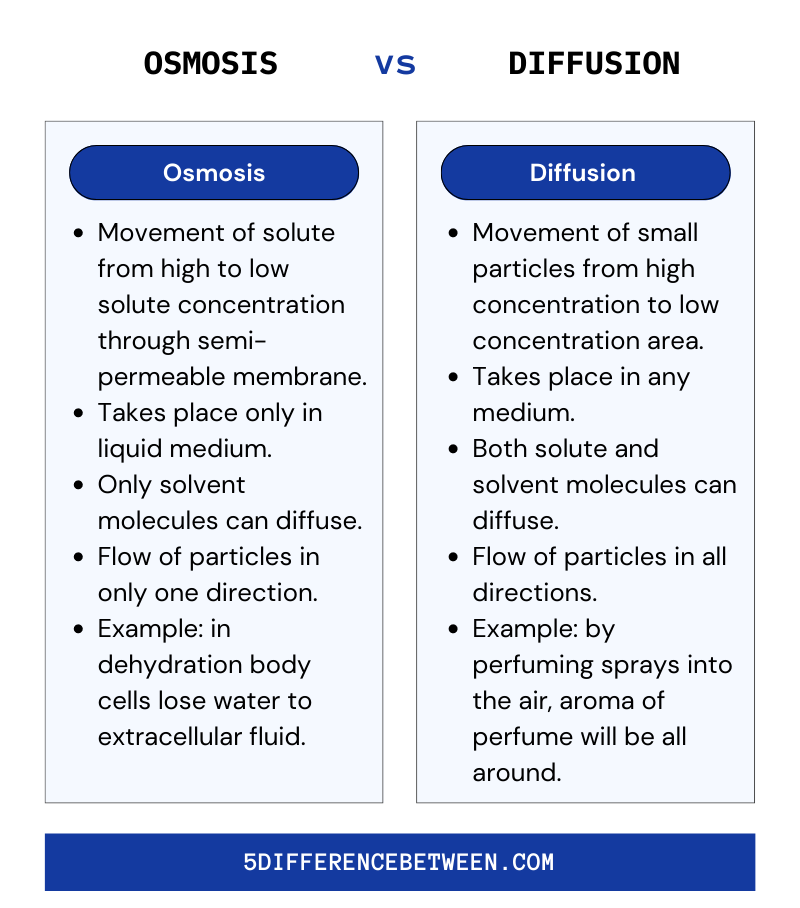
So if you still are not understanding what is the difference between osmosis and diffusion, Then to be precise, the movement of a solvent, such as water, through a semipermeable membrane toward a region of higher solute concentration is referred to as osmosis. Diffusion is the movement of molecules from a high concentration area to a low concentration area. The primary distinction between osmosis and diffusion is that osmosis is the movement of water, whereas diffusion is the movement of any molecule.